Researcher finds help for Alzheimer's-associated agitation with new FDA-approved treatment
A Saint Louis University researcher was instrumental in developing the first and only Food and Drug Administration (FDA) approved treatment for agitation associated with Alzheimer's dementia.
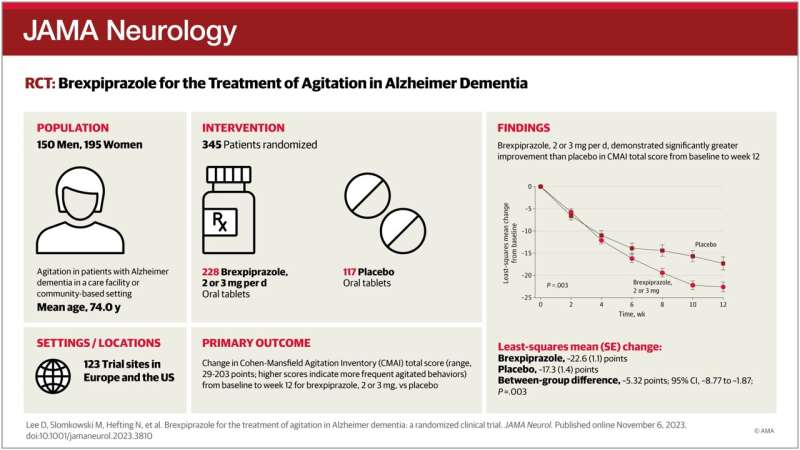
29 jan 2024--In a paper published in JAMA Neurology, senior author and the inaugural Henry & Amelia Nasrallah Endowed Professor and Director of Geriatric Psychiatry at Saint Louis University George T. Grossberg, M.D., and colleagues shared the results of a national clinical trial. They discovered that REXULTI, also called brexpiprazole, significantly reduced agitation in patients with Alzheimer's disease and was well tolerated with few side effects.
Earlier this year, brexpiprazole became the first FDA-approved treatment of agitation-associated Alzheimer's dementia.
Of the 6.7 million people 65 and older in the US with Alzheimer's dementia, multiple studies show that about half or more develop agitation.
Agitation associated with Alzheimer's dementia may include activities like restlessness or more aggressive behavior, like screaming, destroying objects, or fighting. Frequent and severe behavioral symptoms can be extremely distressing to the person with Alzheimer's disease, as well as their families and caregivers.
Antipsychotic drugs are commonly prescribed "off-label" to treat symptoms like aggression and agitation. While these antipsychotics seem to show a modest benefit in treating aggression in the short term, they have adverse effects and other health risks that limit their use over more extended periods.
"When patients with Alzheimer's dementia develop agitation symptoms, they can become increasingly difficult to manage," said Grossberg, who is also director of geriatric psychiatry at SLU. "I'm encouraged by the findings of this study which show that brexpiprazole is an effective and well-tolerated medication that can treat the often-debilitating symptoms of agitation associated with dementia due to Alzheimer's disease."
In the multicenter Phase 3 clinical trial, researchers evaluated the efficacy and safety of brexpiprazole, a medication used for the treatment of major depressive disorder and schizophrenia, for patients with agitation associated with Alzheimer's.
The clinical trial was a 12-week, double-blind, placebo-controlled, fixed-dose, parallel-arm trial that enrolled 345 participants at 123 clinical trial sites in Europe and the United States.
Investigators enrolled participants between the ages of 55 and 90 with a diagnosis of probable Alzheimer's disease and clinically significant symptoms of agitation who lived in a care facility or community-based setting.
Participants were randomly assigned to receive the study drug or a placebo. To participate in the clinical trial, participants had to be stable and have a caregiver who could comply with the study procedures.
"It can be extremely challenging to care for patients with Alzheimer's disease," said Grossberg. "Having new medications to help patients who are suffering will enormously benefit patients, health care providers, and those caring for their loved ones."
More information: Daniel Lee et al, Brexpiprazole for the Treatment of Agitation in Alzheimer Dementia, JAMA Neurology (2023). DOI: 10.1001/jamaneurol.2023.3810