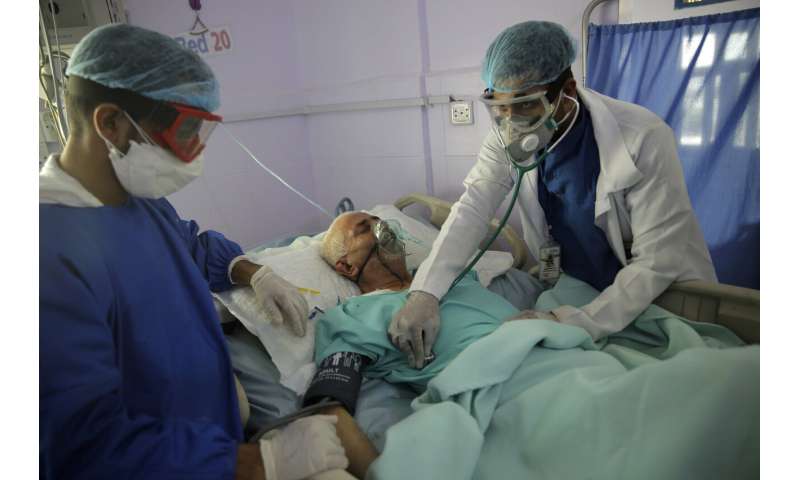
Urgent need for coronavirus testing in care homes, suggests study

A team of academics from the UK Dementia Research Institute (UK DRI) has undertaken a coronavirus outbreak investigation in four London nursing homes.
22 Jun 2020--The research, which has not yet been peer-reviewed, showed 26 percent of residents across four nursing homes died between March and May, three times the rate in previous years.
High rates of coronavirus infection (40 percent) were detected, and 60 percent of those infected were asymptomatic or had atypical symptoms.
Specific, tailored measures are needed to manage coronavirus infection in care homes, say the research team, including comprehensive and repeated testing
The researchers worked with GPs, infectious diseases experts, a geriatric clinical outreach team and Local Authority colleagues to undertake a coronavirus outbreak investigation in four London nursing homes. The multidisciplinary team was convened by Hammersmith & Fulham Council's Director of Public Health, after large numbers of residents at one of the homes became unwell at the end of March.
Using a robotic testing platform developed by the UK DRI team earlier in the month, 313 residents and a selection of staff were tested for the virus to assess infection rates and identify the particular challenges facing nursing homes in controlling an outbreak. A report from the investigation, authored by UK DRI researchers and published as a preprint on MedRxiv, found that comprehensive and repeated testing of both residents and staff are vital to controlling infection rates in nursing homes.
Four-hundred-sixteen-thousand people in the UK live in care homes, where COVID-19 has led to very high levels of illness and death. The World Health Organisation has estimated that as many as half of all COVID-19 deaths in Europe are in care homes. However, little information is available to understand the details of outbreaks in the care home context.
Many people infected without showing symptoms
The team, which included academics from the UK DRI's Care Research and Technology centre based at Imperial College London and the University of Surrey, tested the residents at two time points a week apart, with systematic testing starting on 15 April. Clinical and demographic information was also collected and studied.
A sample of asymptomatic nursing home staff in a variety of roles were also tested to clarify the role that unrecognised staff infections may play in viral transmission. Test results were reported back to residents and care staff promptly to inform decisions on how to minimise further infection.
All of the nursing homes involved had experienced an outbreak of COVID-19, and together saw 103 deaths across a total population of 394 residents between 1 March and 1 May. This was three times the death rate in previous years. Around half of the deaths were attributed to COVID-19 on the death certificate, and over half of those who died had dementia.
Care homes suffered significant outbreaks
Testing found that 40 percent of residents were positive for coronavirus, with 60 percent of these being asymptomatic or displaying only atypical symptoms. Four percent of the asymptomatic staff sample tested positive, suggesting that staff are likely to be a factor in transmitting infection. Viral sequencing showed multiple distinct clusters of SARS-CoV-2 in the same nursing homes.
The study found that COVID-19 often presented in an atypical way—if at all—in nursing home residents: respiratory symptoms such as a cough or shortness of breath often existed already in patients and hallmark signs such as fever were often absent. Many residents, particularly those with the highest care needs, displayed no symptoms at all in the lead up to death. This means that usual approaches to infection control that rely on the identification of symptoms, contact tracing and isolation, are ineffective.
Professor David Sharp, joint senior author of the investigation and Director of the UK DRI's Care Research and Technology Centre based at Imperial College London and the University of Surrey, said:
"We know that many nursing homes have suffered significant outbreaks but until now there's been little data on prevalence, infection rates and what's needed in this setting to combat the virus. The number of deaths in the four homes we studied shows just how pressing an issue this is.
"Dealing with an outbreak of this nature in a nursing home presents many challenges. We found that a very high proportion of those testing positive had no symptoms, or different symptoms from those expected. This makes it extremely difficult for staff to recognise illness and take appropriate measures to protect those they care for. Universal and systematic testing of residents and staff is needed across nursing homes if infection is to be contained.
"The logistics of mass testing are challenging and nursing homes will likely need increased resource to be able to do it well. We were fortunate to be working with a fantastic team of GPs, infectious diseases experts, a geriatric clinical outreach team and colleagues from the Council. This was a prime example of people from different fields and settings coming together and acting quickly as a team to help tackle the virus to protect the most vulnerable."
Testing vital to understanding the virus
Professor Bart de Strooper, Director of the UK Dementia Research Institute, said:"This investigation demonstrates how systematic testing is vital to our understanding of the virus, how it is transmitted, and how it can be contained. By recognising that many people in nursing homes are becoming unwell, often with no symptoms, infection control measures can be adapted, having an immediate impact.
"The UK DRI exists to transform the lives of people with dementia, so when we saw an opportunity to deliver a rapid project to benefit vulnerable older people, we mobilised resource and technology to make sure we could get results fast.
"Our team had already created a new high-throughput coronavirus testing platform in just nine days last month and the speed of this project has been no less staggering. A little over a week after the first conversation with colleagues about introducing testing in nursing homes, every resident had been tested in four homes."
Dr. Nicola Lang, Acting Director of Public Health and Consultant in Public Health Medicine for Hammersmith & Fulham Council, said:"In response to the care home outbreaks, we rapidly drew together what now feels like a unique collaboration between GPs, virology, elderly medicine, frailty matrons, infectious diseases teams, academia, and paediatric infectious diseases and epidemiology teams.
"They helped me manage the outbreaks with the latest advice, enabling us to test all our residents twice, and also start care home staff testing.
"This rapid testing informed the actions we took with nursing home colleagues, and strengthened our infection control and isolation procedures.
"The legacy is a mature partnership which has already created guidance for many types of COVID-19 situations with elderly patients."
Alarming findings
Fiona Carragher, Director of Research and Influencing for Alzheimer's Society, which part-funded the study through its support of the UK DRI, said:
"This is just a snapshot of the devastation that coronavirus is wreaking in care homes. While a small study, it is alarming that there were three times more deaths than usual, with over half of these being people with dementia. Additionally, over 60 percent of residents who tested positive were asymptomatic, or didn't display the symptoms we're associating with COVID-19.
"At least seven in ten people in care homes have dementia, and it's clear that to keep them safe, we need regular, systematic testing of all residents and staff regardless of symptoms.'
"Our funded researchers have done excellent work to understand the reality of COVID-19 and dementia on the frontline, laying the groundwork for more extensive studies to build on, so we can develop a truly evidenced based approach to keeping people with dementia and all care home residents safe during the pandemic."
Dr. Carol Routledge, Director of Research at Alzheimer's Research UK, which part-funded the study through its support of the UK DRI, said:
"We urgently need to understand the way that COVID-19 is spreading in care homes so we can better protect residents, staff, and their families.
"With over two thirds of people in care homes living with dementia, it's great to see dementia researchers joining efforts to understand the COVID-19 outbreak in these facilities. Recent studies suggest that people with dementia are at higher risk of severe COVID-19 symptoms, and this is an additional source of worry for them and their families.
"The government must be doing all it can to limit infections and prevent further COVID-19 deaths in care homes.
"While this study has collected important data relevant to an ongoing health crisis, the findings have not yet been reviewed by outside experts. This peer review, an important step in medical research, will also be vital for helping to inform government policy."
More information: Neil SN Graham et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes, (2020). DOI: 10.1101/2020.05.19.20105460
Provided by Imperial College London