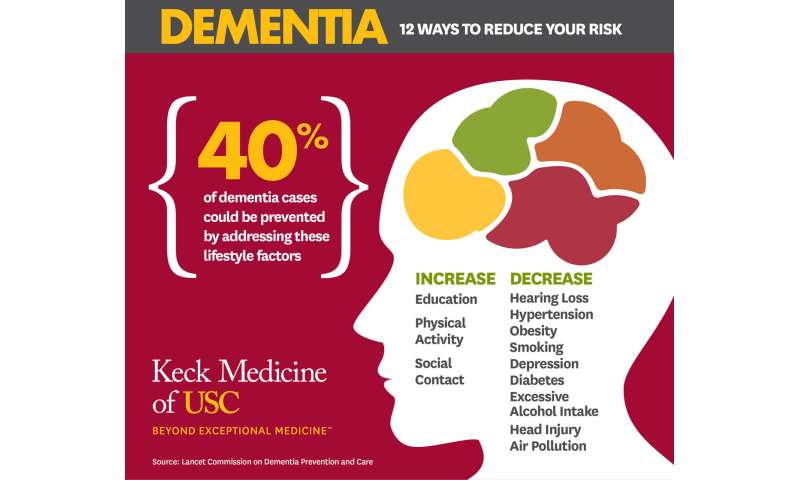
Forty percent of dementia cases could be prevented or delayed by targeting 12 risk factors throughout life

Modifying 12 risk factors over the lifecourse could delay or prevent 40% of dementia cases, according to an update to The Lancet Commission on dementia prevention, intervention, and care, which is being presented at the Alzheimer's Association International Conference (AAIC 2020).
30 july 2020--Combined, the three new risk factors are associated with 6% of all dementia cases—with an estimated 3% of cases attributable to head injuries in mid-life, 1% of cases to excessive alcohol consumption (of more than 21 units per week) in mid-life, and 2% to exposure to air pollution in later life.
The remaining risk factors are associated with 34% of all dementia cases. The factors associated with the greatest proportion of dementia cases in the population are less education in early life, hearing loss in mid-life, and smoking in later life (7%, 8%, and 5%, respectively).
Led by 28 world-leading dementia experts, the report builds on the 9 risk factors identified in the 2017 Lancet Commission, and provides an up-to-date analysis of the best evidence on the prevention of dementia. The new report calls for nations and individuals to be ambitious about preventing dementia and lays out a set of policies and lifestyle changes to help prevent dementia.
Worldwide around 50 million people live with dementia, and this number is projected to increase to 152 million by 2050, rising particularly in low-income and middle-income countries (LMIC) where around two-thirds of people with dementia live. Dementia affects individuals, their families, and the economy, with global costs estimated at about US$1 trillion annually.
In certain countries, however, the proportion of older people with dementia has fallen, probably due to improvements in education, nutrition, health care, and lifestyle changes, demonstrating the possibility of reducing dementia through preventative measures.
"Our report shows that it is within the power of policy-makers and individuals to prevent and delay a significant proportion of dementia, with opportunities to make an impact at each stage of a person's life," says lead author Professor Gill Livingston, University College London, UK. "Interventions are likely to have the biggest impact on those who are disproportionately affected by dementia risk factors, like those in low- and middle-income countries and vulnerable populations, including Black, Asian and Minority Ethnic communities."
Professor Livingston continues, "As societies, we need to think beyond promoting good health to prevent dementia, and begin tackling inequalities to improve the circumstances in which people live their lives. We can reduce risks by creating active and healthy environments for communities, where physical activity is the norm, better diet is accessible for all, and exposure to excessive alcohol is minimized."
To address dementia risk, the authors call for 9 ambitious recommendations to be undertaken by policymakers and by individuals:
- Aim to maintain systolic blood pressure of 130 mm Hg or less in midlife from around age 40 years.
- Encourage use of hearing aids for hearing loss and reduce hearing loss by protecting ears from high noise levels.
- Reduce exposure to air pollution and second-hand tobacco smoke.
- Prevent head injury (particularly by targeting high risk occupations and transport)
- Prevent alcohol misuse and limit drinking to less than 21 units per week.
- Stop smoking uptake and support individuals to stop smoking (which the authors stress is beneficial at any age).
- Provide all children with primary and secondary education.
- Lead an active life into mid, and possibly later life.
- Reduce obesity and diabetes.
These actions are especially important in LMICs where dementia rates are rising more rapidly than in high-income countries. This is a result of increasing life expectancy, and a higher frequency of certain dementia risk factors—such as lower rates of education; high rates of hypertension, obesity, and hearing loss, and rapidly growing rates of diabetes.
Based on their past model including 9 risk factors, the authors estimated that many more cases of dementia could be prevented in LMICs, compared to globally. While globally the 9 risk factors were estimated to contribute to 35% of all dementia cases, in China they might account for 40% of cases, 41% in India and 56% in Latin America.
The authors warn that estimates could be even higher, as they used conservative estimates for the prevalence of these risk factors in these populations, and because they do not account for the three new risk factors. The authors also note that nearly all the evidence for dementia is from studies in high-income countries, so risks might differ for LMICs and interventions might require modifying to best support different cultures and environments.
The authors note that the modeling for their prevention estimates globally and in LMICs assumes that there is a causal relationship between risk factors and dementia, but were careful to only include risk factors with strong evidence for a causal link.
Report co-author, Professor Adesola Ogunniyi, University of Ibadan, Nigeria, says: "In low- and middle-income countries, the higher prevalence of dementia risk factors means an even greater proportion of dementia is potentially preventable than in "higher-income countries". In this context, national policies addressing dementia risk factors, like primary and secondary education for all and stopping smoking policies, might have the potential for large reductions in dementia and should be prioritized. We also need more dementia research coming from low- and middle-income countries, so we can better understand the risks particular to these settings."
In the final section of the report, the authors advocate for holistic and individualized evidence-based care that addresses physical and mental health, social care, and support that can accommodate complex needs. Keeping people with dementia physically healthy is important for their cognition but they often have other illnesses which they may struggle to manage on their own, resulting in potentially harmful preventable hospitalisations.
They note that people with dementia are particularly at risk from COVID-19 (due to age and having pre-existing illnesses, such as hypertension), and that physical-distancing measures can be challenging for dementia patients, who may find it difficult to adhere to the guidelines or distressing to be unable to have contact with carers and family. The authors call for people with unknown COVID-19 status to not be admitted to care homes to protect the existing residents, regular testing of staff and asymptomatic as well as symptomatic residents when there is exposure, not moving staff or residents between homes, and more research into how to protect dementia patients during the current pandemic and future public health emergencies.
More information: Gill Livingston et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. Published:July 30, 2020 DOI: doi.org/10.1016/S0140-6736(20)30367-6